Pristine Seas
Inspiring some of the largest marine reserves in the world.
Without the ocean, life would be impossible. It provides food, livelihoods for billions of people, and regulates the climate. But the ocean is under threat from overfishing, global warming and pollution.
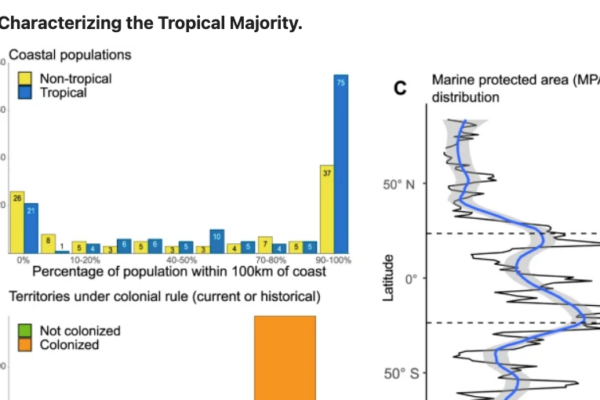
Yet today, only 8% of the ocean is somehow protected – and less than 3% is fully protected from fishing and other damaging activities.
Pristine Seas works with local communities, Indigenous Peoples, government and partners to protect our ocean, but also areas that have been somehow degraded by human activities, so they can bounce back. Marine life thrives in these marine protected areas and provides multiple benefits to people, from food and coastal protection to jobs and economic revenue.
Where we work
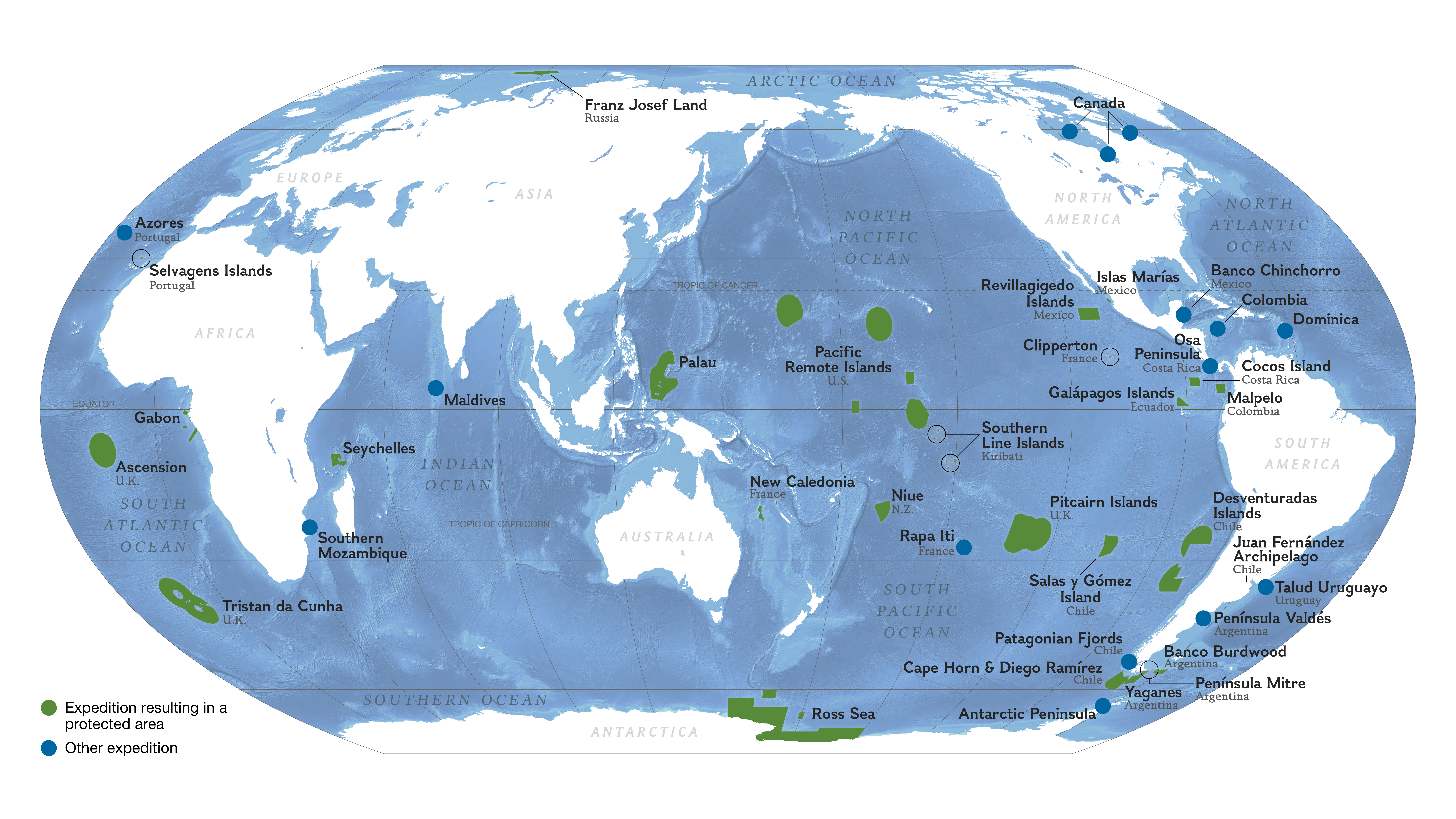
Since 2008, Pristine Seas has carried out 44 expeditions to over 30 places, 27 of which have since been protected, covering a total area of more than 6.6 million square kilometers.
The Global Expedition
In 2023, National Geographic Pristine Seas launched a bold new conservation effort: The Global Expedition. The Pristine Seas team of scientists, policy experts, and filmmakers, will spend the next five years exploring the tropical Pacific aboard the M/V Argo, a 130-foot customized research vessel and media center, with the goal of supporting communities and governments in their efforts to protect the ocean. The Argo is Pristine Seas’ modern-day equivalent of Jacques Cousteau’s famous ship Calypso, but with an ocean conservation purpose.
Field Notes
Explore updates from the field from the Pristine Seas team.

Year Two of Pristine Seas: The Global Expedition Launches in Palau
Pristine Seas returns to Palau to conduct marine surveys, bringing with them Nat Geo Education team members for a special student outreach.

Pristine Seas: The Global Expedition Conducts Nationwide Survey in the Federated States of Micronesia
Pristine Seas with Blue Prosperity Micronesia are invited to conduct a nationwide assessment of the marine ecosystems including remote atolls, seamounts and reefs in the Federated States of Micronesia.

Pristine Seas: The Global Expedition Surveys Four Coral Atolls in the Marshalls Islands
Pristine Seas is invited to the Marshall Islands on its fourth stop in The Global Expedition to survey Bikar and Bokak atolls in support of Reimaanlok. The team will also survey Bikini, the site of 23 nuclear tests, and Rongerik atolls.

Pristine Seas Returns to Niue on the Global Expedition
Pristine Seas is invited to return to Niue to monitor changes in their marine reserve, since our original survey in 2016. The results will support the management of the reserve and Niue’s ocean conservation efforts.

The Global Expedition Studies Sharks in Tongareva, Cook Islands
The Pristine Seas team makes its way to the shark-filled waters of Tongareva, Cook Islands on the second leg of its Global Expedition with Cook Islands-based nonprofit organization Sharks Pacific and Explorer Jess Cramp.

Pristine Seas Launches the Global Expedition in Kiribati
Pristine Seas: The Global Expedition launches a five year voyage in the Pacific by returning once more to the southern Line Islands in Kiribati – the location that launched the Pristine Seas program.
A closer look
Our team of scientists, filmmakers and policy experts have traveled across the ocean — from the poles to the tropics — to inspire the creation of marine protected areas. Take a closer look at some success stories from around the world.
Tristan da Cunha
Juan Fernández Archipelago
Revillagigedo Islands
Niue
Scientific research
Pristine Seas team members have collectively published more than 200 scientific publications in peer-reviewed journals ranging from Nature, Science Advances, PLOS One and more.
Latest stories
Our impact is often featured in the news. Here are some of the most recent stories about the work the Pristine Seas team is doing around the world.



Leveraging world-class expertise
Founder

Enric Sala
Enric Sala is a former university professor who saw himself writing the obituary of ocean life, and quit academia in 2008 to become a full-time conservationist as National Geographic Explorer in Residence. He founded and leads National Geographic Pristine Seas, a project that combines exploration, research, media, economics and policy – working with local communities, Indigenous Peoples and governments to protect vital places in the ocean. To date, Pristine Seas has helped to create 26 of the largest marine reserves on the planet, covering an area of over 6.5 million square km. Enric has received numerous awards, including 2008 World Economic Forum’s Young Global Leader, 2013 Explorers Club Lowell Thomas Award, 2018 Heinz Award in Public Policy, National Geographic Hubbard Medal, Albert I Grand Medal, and Officer of the Order of San Carlos of Colombia. He is a fellow of the Royal Geographical Society.
Business Operations
Patrick Hare
Sam Mandl
Katherine Warren
Katie Warren manages team logistics, programmatic activities, and supports communications across the program. She is an environmental scientist by training and a conservationist committed to preserving and restoring marine ecosystem health. She holds a B.S. in Biology from the University of New England and a M.S. in Environmental Contamination and Toxicology from the University of the Basque Country. Before joining the Pristine Seas team, Warren conducted and managed research activities worldwide, including characterizing harmful algal blooms in the Biscay province of Spain and exploring the population genetics of macroalgae across the North Atlantic. After spending time in the field, Warren joined the Operations team where she now applies her experience to the program so expeditions run smoothly.
Siena Inaba
Expeditions
Paul Rose
Sydney McDonald
Ryan Jenkinson
Doug Simpson
Mike Barnaby
Keiron Fraser
Research
Alan Friedlander
Over the past 40 years Alan Friedlander has spent over 10,000 hours underwater—among coral reefs, to depths of thousands of meters, and at both poles. He has been involved with the project since its inception and is also a researcher at the Hawai‘i Institute of Marine Biology. Friedlander leads research efforts to help understand and conserve the last wild places in the ocean and his work on marine conservation ranges from small-scale, community-managed areas to some of the largest protected areas on the planet. He has authored over 200 scientific publications. Friedlander received a Master’s degree in Oceanography from Old Dominion University, a Ph.D. from the University of Hawai‘i, and was a National Research Council postdoctoral associate. He is a fellow of both the Royal Geographical Society and the Explorers Club.
Molly Timmers
Molly Timmers is a marine ecologist who uses both traditional survey and cutting-edge molecular techniques to better understand the biodiversity of marine organisms across spatial and environmental gradients. Prior to National Geographic, she served as a marine scientist for 20 years with NOAA’s Pacific Reef Assessment and Monitoring Program researching coral reefs across more than 50 islands and atolls throughout the Pacific Ocean. She leads Pristine Seas’ eDNA initiative to fill knowledge gaps in the biodiversity and distribution of inconspicuous marine life from the shallows to the deep. She has a B.S. in Biology from Hamilton College, a M.S. in Conservation Biology and Environmental Science from the University of Hawaiʻi Hilo, and a Ph.D. in Zoology from the University of Hawaiʻi Mānoa.
Kike Ballesteros
Kat Millage
Juan Mayorga
Juan Mayorga is a marine scientist working to leverage emerging Earth monitoring technologies and datasets to catalyze ocean conservation. His work combines data science, marine ecology, economics, and conservation planning to distill actionable insights and support the creation of marine protected areas. Mayorga leads Pristine Seas’ partnership with Global Fishing Watch and the Environmental Markets Lab (EmLab) at the University of California, Santa Barbara, where he is an associate researcher. This collaboration has been instrumental in accelerating ocean protection by producing novel scientific research including the first high-resolution analysis of industrial fishing worldwide. His most recent work involves developing a state-of-the-art conservation prioritization framework to identify the most critical places in the ocean to protect biodiversity, safeguard carbon stocks, and improve fisheries yields. Mayorga manages Pristine Seas’ scientific database which brings together a wide array of scientific methods used by the team to explore and assess ocean life during our expeditions. At sea, he also leads our work exploring the twilight zone with mesophotic baited remote underwater video stations. Mayorga holds a BEng in environmental engineering from Universidad de Los Andes in Colombia and a master’s in environmental science and management from UC Santa Barbara. He’s a member of the National Ocean Protection Council scientific advisory team, the Ocean Panel Expert Network, and Global Fishing Watch’s Science Advisory Committee.
Chris Thompson
Chris Thompson is a marine ecologist and zoologist with a keen interest in anything to do with the natural world. The chief focus of his work is the pelagic ocean and the seabirds strongly linked to it. He has taken part in over twenty research expeditions working in locations from his backyard in Western Australia to the remote tropics, arctic, and sub-Antarctic. In this time he has developed sampling skills including deploying seabed, deep-sea and pelagic camera systems, surveying seabirds, tagging sharks, and conducting visual census’ of fishes, corals, and invertebrates. He completed his PhD at the University of Western Australia; in which he explored the impact of humans on global pelagic wildlife assemblages and what remote places can teach us about intact ecosystems. Through his work, Chris hopes to better understand our natural world, the drivers shaping it, and what we can do to conserve it.
Whitney Goodell
Media
Scott Ressler
Brian Newell
Brian Newell is the Senior Editor for the National Geographic Pristine Seas team, producing and editing documentary films to help inspire ocean conservation and designing post-production technology solutions for the video production team. He has edited two award-winning feature documentaries at National Geographic: Into the Okavango (2018) and The Last Ice (2021). Brian graduated with a B.A. in film studies in 2005 and worked as a post-production supervisor and editor on shows for History, Discovery, Travel, PBS, and others before joining National Geographic.
Christian Partenio
Christian Partenio works with the media team, editing and assisting with the production of short form and long form videos to advance the goals of the team at large. Christian worked with Disney Broadcast (NGC) for 3 years prior to joining Pristine Seas, working in the post-production of episodic docuseries as well as producing and editing various special projects. Before that, he worked as a corporate media shooter, producer and editor. He holds a degree in Digital Media and Design from the University of Connecticut.
Mags Miller
Mags Miller is a TCK (Third Culture Kid) who grew up in Nigeria, Tanzania and Zimbabwe. Over the past 18 years she has served as a Producer, Director and Show Runner for National Geographic TV, PBS, and numerous Discovery Networks. She’s embedded with the USMC in Afghanistan, hung with Orangutans in Borneo, navigated through 25 hoarder’s homes, directed a variety of personalities and has eaten delicious food in amazing destinations. Her love of visual storytelling comes from a desire to create compassionate yet powerful content to help shed light on important global issues. Miller holds a Masters in Film and TV from American University.
David Taylor
Manu San Félix
Steve Spence
Liz Flamenbaum
Christine Fitzpatrick
Jon Betz
Sam Deleon
Melanie Lippert
Melanie Lippert assists with the pre- and post-production elements for Pristine Seas documentaries, helping to highlight and protect marine ecosystems in regions such as Dominica, Argentina and the Marshall Islands. Prior to Pristine Seas, she worked at the National Wildlife Federation as a Communications Fellow and for the Cheetah Conservation Fund in Namibia and Somaliland. She also worked at National Geographic Channel as a Production Assistant, helping with shows such as Clotilda: Last American Slave Ship and Gorongosa: Paradise Reborn. Lippert holds a B.A. in Biology and Journalism from the University of Richmond.
Hamza Kiyani
Alex Verville
Tess Goldhagen
Tess Goldhagen is a producer who has worked on multiple Pristine Seas documentaries, including the feature-length film The Last Ice. Her work as a shooter and field producer on Pristine Seas expeditions have taken her around the world—from the Arctic to the Pacific—and has allowed her to tell stories about the resilience of healthy marine ecosystems and the communities that depend on them. Before working at National Geographic, she worked at non-profit organizations based in the United States, Ecuador and Uganda focused on social and environmental rights. Goldhagen holds a Bachelor’s degree in international development from McGill University.
Yaqui Mennes Pine
Mary Nail
Mary Nail supports the post-production of Pristine Seas’ documentaries. While she has edited content across many genres and platforms, her heart has always belonged to documentary filmmaking. She is thrilled to be creating compelling films to protect our planet’s oceans with the Pristine Seas team. Nail holds a Bachelor’s degree in history from Hendrix College.
Strategy
Dan Myers
Jenny Miller
Kevin Chand
Prior to joining the National Geographic Society, Kevin Chand served as the Senior Legal Adviser at the Permanent Mission of Vanuatu to the United Nations. Chand played a prominent role in the campaign to take the issue of climate change to the International Court of Justice, co-facilitating the negotiations of this resolution, leading efforts to develop and implement a legal and political strategy for the initiative. While at the UN, he actively contributed to the PSIDS group in the BBNJ negotiations.
Chand began his career in ocean law and policy in Fiji focused on the intersection of customary marine tenure systems, Indigenous fishing rights, and national legal systems. Previously he worked with regional organizations in the Pacific including the Pacific Islands Forum Fisheries Agency and the Pacific Community. Chand has taught at Stanford at and was based at the Stanford Center for Ocean Solutions.
Chand has an LLM from Stanford Law School, a Master of Environmental Management from the University of Queensland and a Bachelor of Law from the University of the South Pacific.
Courtney Lorey
Mia Rimon
Mia Rimon is a Pacific expert who has been working in senior roles in Government and Pacific Regional Organizations for more than two decades years. Prior to joining the Pristine Seas team as Pacific Advisor, Rimon was the Regional Director for Melanesia for the Pacific Community (SPC). In Melanesian countries she has played a lead role in developing National Ocean Policies, in resourcing and supporting completion of Marine Spatial Plans and implementation of National Ocean Policies in the Solomon Islands, Vanuatu, and Papua New Guinea. She has advised Pacific Island Countries and Territories on policy development and served as key advisor to the Chairman of the Vanuatu Public Service Commission. Mia founded the Youth@Work programme providing employment and training to unemployed and uneducated youth in the Solomon Islands. Mia served the Pacific Community for 17 years as a member of the Senior Leadership Team of SPC. Rimon holds a Master’s degree with Distinction in Public Policy from Flinders University in Australia and is a recognized regional expert in Gender Based Violence (GBV), having completed national studies and interventions on GBV in Kiribati and the Solomon Islands.
Taholo Kami
Taholo Kami is a Tongan born, resident of Fiji and was raised in Papua New Guinea. He has advised Pacific Island Governments, International and regional organizations as well as civil society and church networks. Previously he was the regional Director for IUCN Oceania, the Special Representative for Oceans for Fiji and he managed the Small Islands Developing States Network in UNDESA, New York as well as running the biggest early social networking site in the Pacific – the Pacific Islands Kava bowl Forum. He is a founder of WOWS Kids Fiji a charity that supports children with cancer in Fiji, leads the Tongan community and continues to convene and support innovators and emerging leaders. He has an accounting degree from the University of Technology of Papua New Guinea and completed an MBA in marketing/eCommerce as a Fulbright scholar at Vanderbilt University.
Communications
Kirsten Weymouth
Sara Sheehy
Jenelle Eli
Emily Pitts
Emily Kelly
Legal
Jennifer Dill
NGS Education
Chris Hines
Anastasia Cronin
Anastasia Cronin develops and runs education programs that elevate National Geographic Explorers and their work to educators, students, and youth around the globe. She oversees the Explorer Classroom program, a series of interactive, virtual events that broadcast scientists, storytellers and conservationists live into classrooms. In her time at National Geographic, she has worked across the organization in a range of roles including youth leadership, educator outreach, Explorer engagement, and documentary production. Prior to her time at National Geographic, she spent several years doing field work, including wildlife research in Costa Rica and Kenya and archaeological excavations throughout the Eastern US.
Juthapathra Dechanupong

About Pristine Seas
Since 2008, Pristine Seas has helped establish 26 of the largest marine protected areas in the world, covering a total area of 6.6 million square kilometers — more than twice the size of India.
With support from
FOUNDING SPONSOR
Blancpain
FUNDING PARTNERS
Bezos Earth Fund, Bloomberg Philanthropies, Lindblad Expeditions–National Geographic Fund, Don Quixote Foundation, Inclusive Capital Partners Foundation, The Campbell Foundation, Waitt Foundation, Oracle, Dutch Postcode Lottery, LGT Venture Philanthropy, Philip Stephenson Foundation, Walmart Foundation, The Heinz Family Foundation, Beagle Foundation, Serventi Family Foundation, and other individual donors.
PAST FUNDERS
Allison Bennington, Brook Foundation, Jean and Steve Case, Leonardo DiCaprio Foundation, DAVIDOFF Cool Water, Roger and Rosemary Enrico, Helmsley Charitable Trust, Google, David and Lucile Packard Foundation, Vicki and Roger Sant, and other individual donors.
Photo credits (from top of page): Manu San Félix, Ossie Michelin, SerrNovik/Getty/iStockphoto, Manu San Félix, Enric Sala, Ossie Michelin, Enric Sala (2)